PH, what is PH
Chemicals and their Ph are defined on the PH scale. The pH scale is a measure from zero to 14. It reflects the relative amount of free hydrogen and hydroxyl ions in the water – the more hydrogen ions, the more acidic the product, the higher the number of hydroxyl ions, the more alkaline the product.
Acid products have a low PH and alkaline product have a high PH. The middle number on the scale, 7, is used to describe substances that are neutral in acidity – for example, pure water. Any number on the scale below 7 is acidic, any number above 7 is considered alkaline. Acidic substances are great for removing calcium, rust and minerals. At the same time, alkaline solutions are much more effective at cutting through grease, proteins, oils, dirt and organic items.
How do everyday items fit in the PH Scale
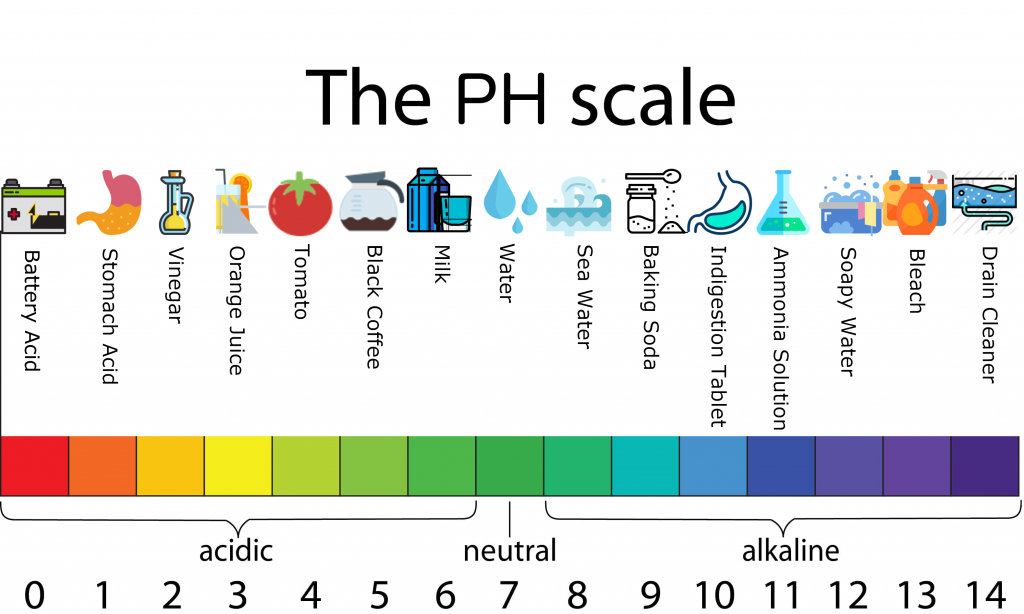
Let’s define the types of Cleaning Chemicals and their PH.
• Acidic Agents
Acidic cleaners usually have mineral acids (like hydrochloric acid) as the active ingredient. They are used to brighten metals or remove mineral deposits and clean oxidized materials. Many bathroom fixture cleaners are acidic, and their purpose is to remove calcium build-ups (water stains/lime deposits) or scaly or corrosive deposits.
• Alkaline Agents
Alkaline cleaners, like degreasers and ammonia, are used to dissolve fats, oils and proteins. Most types of soils will contain these elements, and alkaline cleaning agents will break down these materials and make them easier to remove.
• Neutral Agents
Neutral cleaning agents are called such due to their pH-neutral base. They are useful for dispersing dirt, dust and other debris though an utterly indifferent base. They will have a less cleaning effect than a cleaning agent that falls more to one side of the scale and are ideal for light soil loads.
So what are the typical PH values of chemicals?
• Chlorine bleach
Chlorine Bleach has a pH of between 11 and 13. As an alkaline product, it makes a fantastic whitening and stains removal agent. However, it is relatively corrosive. This means it’s excellent for every hard surface, but you need proper ventilation when using it and be careful about materials and clothes
• Ammonia
Ammonia solutions have a pH of between 11 and 12. Highly alkaline substance, ammonia is a formidable cleaner that can work miracles in removing dirt and grime. Use with proper protective equipment in an area that provides adequate ventilation.
• Mild dish detergent
Mild dishwashing detergents have a pH of between 7 and 8. If you are using dish detergent that’s labelled ‘mild’, this generally means its pH level is sitting just around the middle. Which is perfect for items you’re cleaning daily. Most surfaces won’t get damaged by mild dish soap, and you generally don’t need to wear protection when dealing with products of a neutral acidity.
• Vinegar
Vinegar has a pH of around 3.The acidic properties in vinegar make it perfect for removing tough mineral deposits. Still, it also makes it a wrong choice for some surfaces (notably stone). Vinegar also has the potential to cause real damage if you’re not careful. Protection for your face and hands is a definite must.
• Toilet bowl cleaner
Toilet Bowl Cleaner has a pH of between 1 and 3. Toilet bowl cleaners are extremely acidic but perfect for breaking down minerals and other non-organic nasties that might be lurking in your toilet bowl. You must use these products with extreme caution.
What chemical and PH should I use for cleaning
Woman cleaning pink wall with a microfiber rag, gloves and cleaning spray agent.
For acid-based soils, such as those listed below, use cleaning chemicals with and alkali PH.
- Greasy floor
- Dirty walls
- Cigarette tars
- Engines and tools
- Motor oils, diesel oil, axle grease Cooking oil
- Ventilation hoods
- Ovens
For alkaline-based soils, such as those listed below, use cleaning chemicals with an acid PH.
- Watersports
- Rust
- Calcium deposits Lime deposits
- Inside the dishwasher Toilet bowls
- Shower stalls
- Urinals
Recent Posts
Could the standard of cleaning you are receiving be improved?
If you are not happy with your current cleaning contractor's service, don't just put up with it. Get yourself a company who will go the extra mile! Contact us now to help bring the standard of...
COVID-SECURE Disinfection cleaning & fogging services - Johannesburg. Fogging is a quick way to disinfect large areas and allow them to be returned to everyday use. We use a fogger machine...

